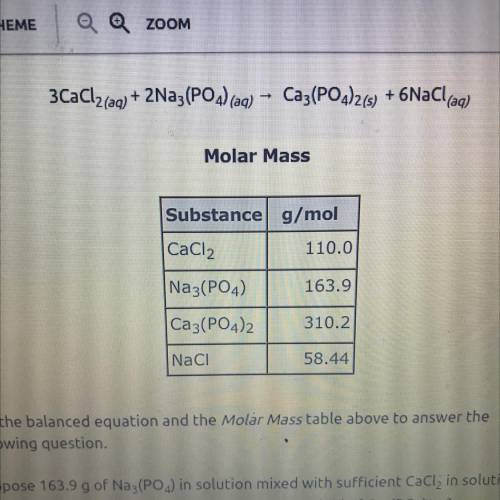
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar...

Chemistry, 16.04.2021 01:00, DragonLovely
3CaCl2(aq) + 2Na3(PO4) (aq)
Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the
following question.
Suppose 163.9 g of Na3(PO4) in solution mixed with sufficient CaCl, in solution
yields 116 g of Ca3(PO4)2(s). What is the percent yield of Ca3(PO4)2(5)?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:10, leo4687
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 21.06.2019 22:30, shantrice1831
Using the periodic table, complete the table to describe each atom. type in your answers. a ? b? c? d? e? f?
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Spanish, 30.06.2019 08:30

Mathematics, 30.06.2019 08:30


Chemistry, 30.06.2019 08:30



History, 30.06.2019 08:30








