
Chemistry, 15.04.2021 23:20, taylormjensen
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> CO2(g) + CF4(g) K = 2.2 x 10^6
Calculate the equilibrium constant for the following reaction at 25oC:
2CO2(g) + 2CF4(g) <--> 4COF2
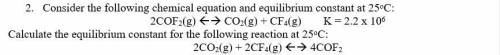

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 23.06.2019 01:00, skatelife8974
What is the chemical name of the compound ti2o3?
Answers: 2

Chemistry, 23.06.2019 03:50, timothymoles
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1

Chemistry, 23.06.2019 05:30, hongkongbrat6840
Astudent made the lewis dot diagram of a compound as shown. mg is written with two dots shown on its top. an o is written on each side of mg. each o has six dots around it. an arrow is shown from one dot on mg toward the vacant space around the o on the right. another arrow is shown from the other dot on mg toward the vacant space around the o on the left. the title of the art is students lewis dot model. what is the error in the lewis dot diagram? an o atom should transfer all its six electrons to mg because the formula is mgo. both electrons of mg should be transferred to one o atom because the formula is mgo. the electrons should be transferred from each o atom to mg because mg has fewer electrons. the number of dots around mg should be four because it has to transfer two electrons to each o.
Answers: 2
Do you know the correct answer?
2. Consider the following chemical equation and equilibrium constant at 25oC:
2COF2(g) <--> C...
Questions in other subjects:






Physics, 15.04.2020 00:26



Mathematics, 15.04.2020 00:26







