
Chemistry, 15.04.2021 18:20, hdjsjfjruejchhehd
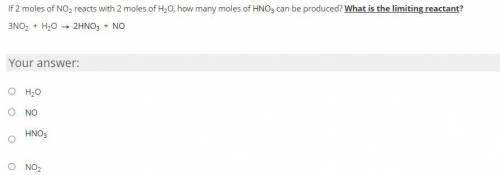
If 2 moles of NO2 reacts with 2 moles of H2O, how many moles of HNO3 can be produced? What is the limiting reactant? -NO LINKS

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 13:00, rome58
Lab reagent, hypothesis test. a reference solution used as a lab reagent is purported to have a concentration of 5 mg/dl. six samples are taken from this solution and the following concentrations are recorded: (5.32, 4.88, 5.10, 4.73, 5.15, 4.75) mg/dl. these six measurements are assumed to be an srs of all possible measurements from solution. they are also assumed to have a standard deviation of 0.2, a normal distributin, and a mean concentration equal to the true concentration of the solution. carry out a significance test to determine whether these six measurements provide reliable evidence that the true concentration of the solution is actually not 5 mg/dl.
Answers: 1


Chemistry, 22.06.2019 17:30, sheazy3709
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
Do you know the correct answer?
If 2 moles of NO2 reacts with 2 moles of H2O, how many moles of HNO3 can be produced? What is the li...
Questions in other subjects:

Mathematics, 12.08.2021 15:40

Mathematics, 12.08.2021 15:40


Physics, 12.08.2021 15:40

History, 12.08.2021 15:40

Mathematics, 12.08.2021 15:40




Geography, 12.08.2021 15:40






