
Chemistry, 15.04.2021 01:20, raffaldarmaki9412
HURRY
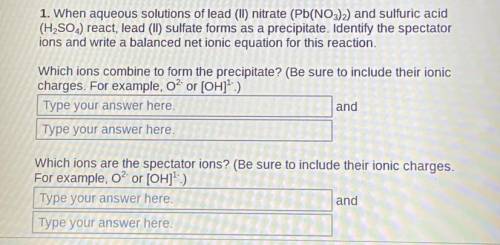
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.
Which ions combine to form the precipitate? (Be sure to include their ionic charges. For example, O² or [OH]¹-)
2. Which ions are the spectator ions? (Be sure to include their ionic charges. For example, O² or [OH]¹-)

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 23.06.2019 07:00, jennnifercrd59jc
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2
Do you know the correct answer?
HURRY
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H2SO4) react, le...
Questions in other subjects:

Mathematics, 07.04.2021 14:40

Mathematics, 07.04.2021 14:40

Computers and Technology, 07.04.2021 14:40

English, 07.04.2021 14:40

History, 07.04.2021 14:40



Mathematics, 07.04.2021 14:40


Physics, 07.04.2021 14:40






