
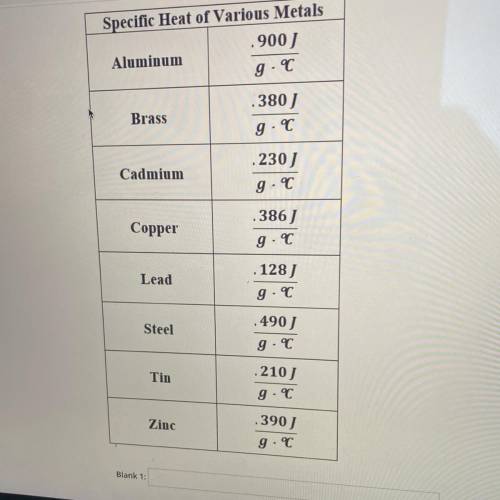
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of water, the water increased temperature from 20.1°C to 35.4°C
If water has a specific heat capacity of 4.18 Jg, determine the unknown metal by calculating it's specific heat. The unknown metal is ___

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, AleciaCassidy
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
Do you know the correct answer?
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of...
Questions in other subjects:

English, 17.09.2021 03:50

English, 17.09.2021 03:50



Mathematics, 17.09.2021 03:50

History, 17.09.2021 03:50

History, 17.09.2021 03:50


Mathematics, 17.09.2021 03:50






