HURRY
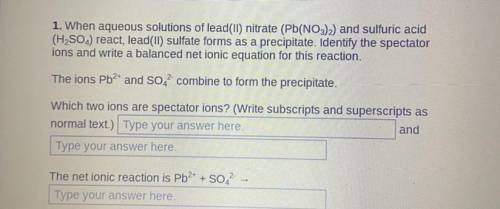
1. When aqueous solutions of lead(II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) reac...

Chemistry, 14.04.2021 17:20, brendacauani12345
HURRY
1. When aqueous solutions of lead(II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) react, lead(II) sulfate forms as a precipitate. Identify the spectator
ions and write a balanced net ionic equation for this reaction.
The ions Pb2+ and SO42- combine to form the precipitate.
Which two ions are spectator ions?
The net ionic reaction is Pb2+ + SO42...

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 19.12.2020 01:00


History, 19.12.2020 01:00

History, 19.12.2020 01:00





Chemistry, 19.12.2020 01:00






