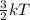Halving the kelvin temperature of a substance will result in:
a. doubling in the molecular motion and collisions between particles and the walls of the container
b. the same molecular motion and collisions between particles and the walls of the container
c. halving the molecular motion and collisions between particles and the walls of the container
d. quadrupling the molecule motion and collisions between particles and the walls of the container

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, rileyeddins1010
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 23.06.2019 00:30, clairebear66
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 05:40, 19youngr
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2

Chemistry, 23.06.2019 06:00, bvbbridesmaid5519
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
Do you know the correct answer?
Halving the kelvin temperature of a substance will result in:
a. doubling in the molecular mo...
a. doubling in the molecular mo...
Questions in other subjects:

History, 03.03.2021 23:00

History, 03.03.2021 23:00




Mathematics, 03.03.2021 23:00

Spanish, 03.03.2021 23:00



Social Studies, 03.03.2021 23:00