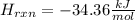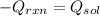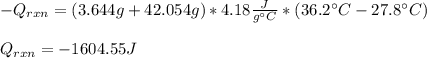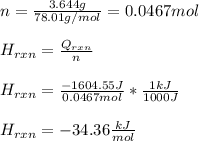Chemistry, 14.04.2021 09:30, ichabella2010
If 3.644 g (78.01 g/mol) is dissolved in 42.054 g of water and the temperature goes
from 27.8°C to 36.2°C, what is the molar Hrxn (kJ/mol)? Assume the solution has s
= 4.18 Hint: what is the mass of the entire solution?
g.°C

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, janetexcoelho
What does the mass of 0.7891 mol of ferric oxide (fe2o3)
Answers: 1

Chemistry, 22.06.2019 12:30, poopybutt541
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 00:20, destromero
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1

Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
Do you know the correct answer?
If 3.644 g (78.01 g/mol) is dissolved in 42.054 g of water and the temperature goes
from 27.8°C to...
Questions in other subjects:

Mathematics, 19.02.2021 22:50





Mathematics, 19.02.2021 22:50

Biology, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50

Mathematics, 19.02.2021 22:50