
Chemistry, 14.04.2021 05:50, carter4026
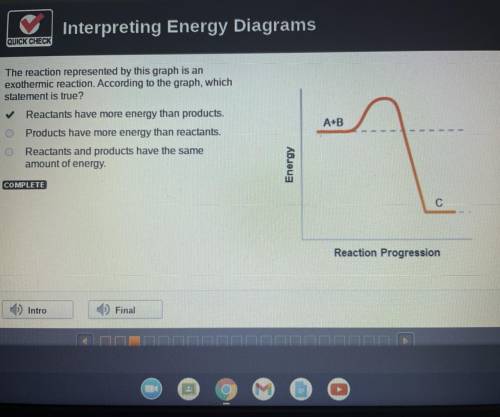
The reaction represented by this graph is an
exothermic reaction. According to the graph, which statement is true?
Reactants have more energy than products.
Products have more energy than reactants.
Reactants and products have the same
amount of energy.
A+B
Energy
COMPLETE
C
Reaction Progression

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, drivinghydra
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:10, dhailyortegacampa131
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
Do you know the correct answer?
The reaction represented by this graph is an
exothermic reaction. According to the graph, which sta...
Questions in other subjects:



Mathematics, 14.12.2020 14:00

Mathematics, 14.12.2020 14:00

History, 14.12.2020 14:00




Computers and Technology, 14.12.2020 14:00

History, 14.12.2020 14:00






