
Chemistry, 14.04.2021 02:40, macattack6276
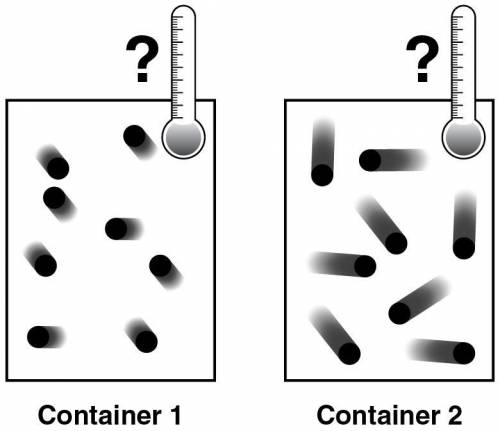
Two containers, illustrated below, are filled with the same amount and the same kind of ideal gas.
Which statement correctly describes the relationship between the contents of the two containers?
Container 1 particles have higher average kinetic energy and higher temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and lower temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and lower temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and higher temperature than Container 2 particles.
Container 1 particles have lower average kinetic energy and higher temperature than Container 2 particles.
Container 1 and Container 2 particles have the same average kinetic energy and the same temperature.
Container 1 and Container 2 particles have the same average kinetic energy and the same temperature.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, amariyanumber1923
19. at high pressures, how does the volume of a real gas compare with the volume of an ideal gas under the same conditions, and why? eman- it is much less because real gas partides are not moving. there is no difference because the gas laws are always obeyed. it is much less because at high pressures the temperature drops. it is much greater because real gas partides take up space.
Answers: 1

Chemistry, 21.06.2019 15:50, leannamat2106
If the mass of the products measured 120g what would the mass of the reactants a. 30g b. 60g c. 120g d. 240g
Answers: 1

Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2
Do you know the correct answer?
Two containers, illustrated below, are filled with the same amount and the same kind of ideal gas....
Questions in other subjects:

Mathematics, 23.05.2021 07:50

Biology, 23.05.2021 07:50

Social Studies, 23.05.2021 07:50






Biology, 23.05.2021 07:50

Social Studies, 23.05.2021 07:50






