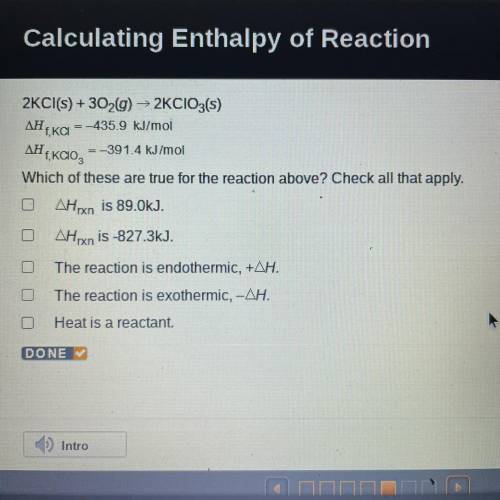
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,<...

Chemistry, 13.04.2021 07:10, Harini5721
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,
Which of these are true for the reaction above? Check all that apply.
AHxn is 89.0kJ.
OAHrxn is -827.3kJ.
The reaction is endothermic, +AH.
O The reaction is exothermic, -AH.
Heat is a reactant.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, corcoranrobert1959
How do dying stars contribute to the formation of planets
Answers: 1


Chemistry, 23.06.2019 08:00, oopsorry
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 10.09.2021 23:30


English, 10.09.2021 23:30







Social Studies, 10.09.2021 23:30






