
Chemistry, 13.04.2021 03:40, ridzrana02
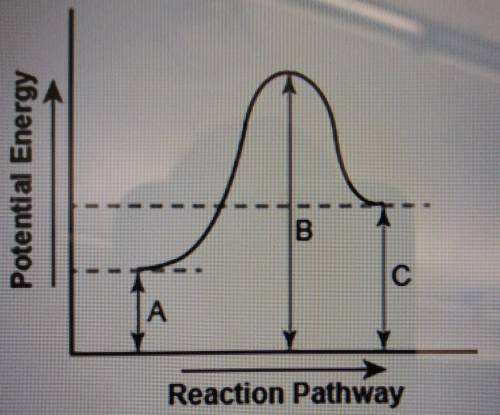
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy B C TA Reaction Pathway Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
Do you know the correct answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy...
Questions in other subjects:

Biology, 20.05.2021 05:20

Social Studies, 20.05.2021 05:20

Mathematics, 20.05.2021 05:20







Mathematics, 20.05.2021 05:20






