Chemistry, 18.09.2019 12:00, babyphoraaaaa
Ascientist observes that after mixing a solid powder into water, the ph of the newly formed solution is 2.7. what can he infer from this observation?
the solution contains an indicator that has a ph of 2.7.
the solid powder contained hydrogen ions.
the ph indicator was used incorrectly.
the powder increased the hydrogen ion concentration of the solution.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Do you know the correct answer?
Ascientist observes that after mixing a solid powder into water, the ph of the newly formed solution...
Questions in other subjects:

Mathematics, 25.08.2019 21:30


Physics, 25.08.2019 21:30

History, 25.08.2019 21:30


Mathematics, 25.08.2019 21:30

Health, 25.08.2019 21:30

Mathematics, 25.08.2019 21:30



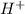 ion in the solution.
ion in the solution.![pH=-\log[H^+]](/tpl/images/0239/6169/cf945.png)
![2.7=-\log[H^+]](/tpl/images/0239/6169/ae8ca.png)
![[H^+]=0.00199 M](/tpl/images/0239/6169/5e039.png)