
Chemistry, 13.04.2021 01:30, mendezmarco2004
A 10.0-mL sample of sulfuric acid, H2SO4, from a battery of an old car is diluted to 100.0 mL, and a 10.00-mL aliquot (portion) of the diluted acid is then titrated with 0.2500 M NaOH solution. If the concentration of H2SO4 in the original battery was 3.25 M, how many milliliters (mL) of the NaOH solution is required to titrate the sulfuric acid present in the 10.0-mL portion of dilute acid solution

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, froyg1234
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1



Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
Do you know the correct answer?
A 10.0-mL sample of sulfuric acid, H2SO4, from a battery of an old car is diluted to 100.0 mL, and a...
Questions in other subjects:

Spanish, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10


Mathematics, 30.10.2020 18:10

History, 30.10.2020 18:10




Mathematics, 30.10.2020 18:20


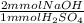 = 6.5 mmol NaOH
= 6.5 mmol NaOH



