
Chemistry, 13.04.2021 01:00, shyiann7910
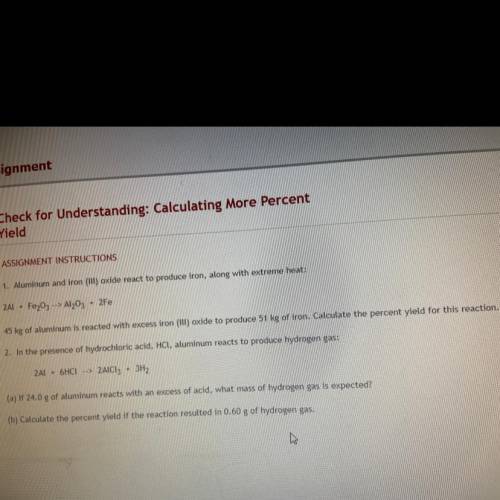
1. Aluminum and iron oxide react to produce iron along with extreme heat 2Al * Fr2O4—> Al2O3+ 2Fe 45 KG aluminum is reacted with excess iron (III) oxide to produce 51 KG of iron. Calculate the percent yield for this reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 12:00, 1963038660
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1


Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
Do you know the correct answer?
1. Aluminum and iron oxide react to produce iron along with extreme heat 2Al * Fr2O4—> Al2O3+ 2Fe...
Questions in other subjects:


Mathematics, 09.11.2020 18:40



Mathematics, 09.11.2020 18:40

Biology, 09.11.2020 18:40

History, 09.11.2020 18:40









