
ASAP PLS
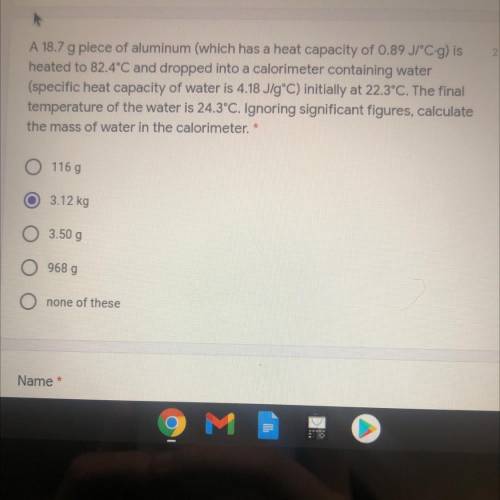
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82.4°C and dropped into a calorimeter containing water
(specific heat capacity of water is 4.18 J/gºC) initially at 22.3°C. The final
temperature of the water is 24.3°C. Ignoring significant figures, calculate
the mass of water in the calorimeter. *

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Do you know the correct answer?
ASAP PLS
A 18.7 g piece of aluminum (which has a heat capacity of 0.89 JPC-g) is
heated to 82...
heated to 82...
Questions in other subjects:

Mathematics, 22.01.2021 23:10

English, 22.01.2021 23:10

Arts, 22.01.2021 23:10


History, 22.01.2021 23:10


Mathematics, 22.01.2021 23:10


Chemistry, 22.01.2021 23:10

English, 22.01.2021 23:10







