
Chemistry, 12.04.2021 18:10, jameskarbar9p8c9d2
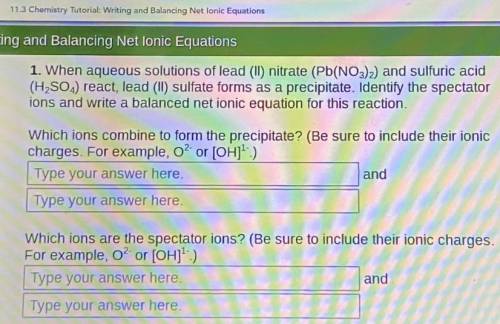
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Do you know the correct answer?
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II)...
Questions in other subjects:

Mathematics, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50




Arts, 11.11.2020 23:50


Mathematics, 11.11.2020 23:50

Mathematics, 11.11.2020 23:50







