
Chemistry, 11.04.2021 23:30, sarahelisabeth444
Help please
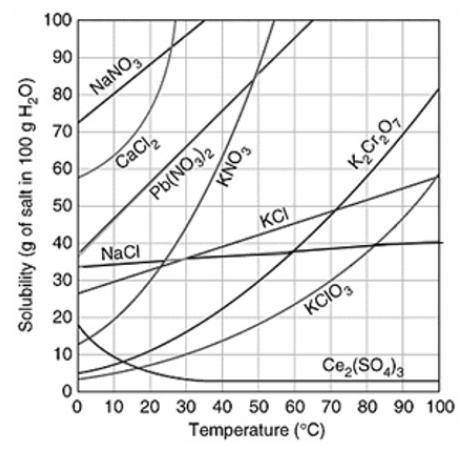
Assuming that the trends continue, which of the following compounds do you predict will have the GREATEST solubility at 120°C?
A.
Ce2(SO4)3
B.
K2Cr2O7
C.
Pb(NO3)2
D.
NaCl

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 17:00, zaehairston78531
What is the nature of the ca-cl bond in a molecule of calcium chloride (cacl2) if the electronegativity value of calcium is 1.0 and that of chlorine is 3.16?
Answers: 1

Chemistry, 22.06.2019 07:30, 10040813
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3
Do you know the correct answer?
Help please
Assuming that the trends continue, which of the following compounds do you predict will...
Questions in other subjects:

Physics, 06.11.2021 02:00

Biology, 06.11.2021 02:00



History, 06.11.2021 02:00











