
Chemistry, 11.04.2021 22:20, andreagrimaldo4
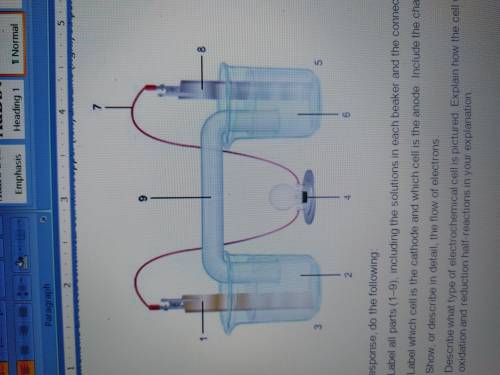
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
In your response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1


Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
Do you know the correct answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
In your re...
Questions in other subjects:




Mathematics, 03.02.2020 22:58

English, 03.02.2020 22:58

History, 03.02.2020 22:58



Mathematics, 03.02.2020 22:58

Mathematics, 03.02.2020 22:58







