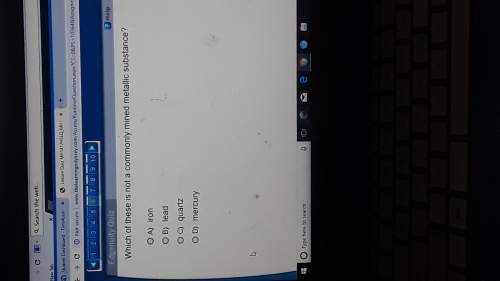
Hydrogen and oxygen react under a specific set of conditions to produce water according to the following equation.
2 H2(g) + O2(g) 2 H2O(g)
(a) How many moles of hydrogen would be required to produce 2.8 mol of water?
(b) How many moles of oxygen would be required?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1


Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 07:30, lifeislove3251
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 1
Do you know the correct answer?
Hydrogen and oxygen react under a specific set of conditions to produce water according to the follo...
Questions in other subjects:

English, 27.10.2020 22:20

Spanish, 27.10.2020 22:20


English, 27.10.2020 22:20




English, 27.10.2020 22:20