
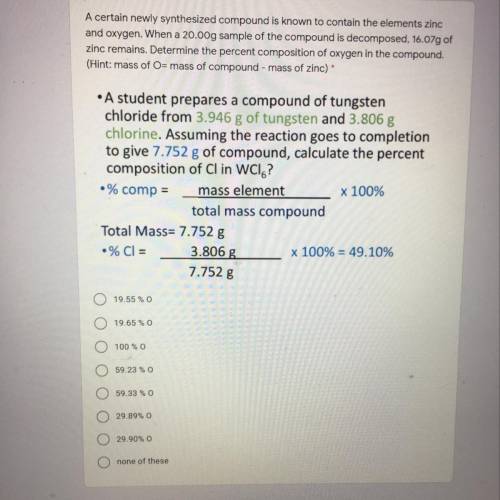
A certain newly synthesized compound is known to contain the elements zinc
and oxygen. When a 20.00g sample of the compound is decomposed, 16.07g of
zinc remains. Determine the percent composition of oxygen in the compound.
(Hint: mass of O= mass of compound - mass of zinc)
Answers are at the bottom someone please help

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 14:30, darkghostmist
What type of reaction fuels the processes seen here?
Answers: 2


Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
Do you know the correct answer?
A certain newly synthesized compound is known to contain the elements zinc
and oxygen. When a 20.00...
Questions in other subjects:


Mathematics, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10


Chemistry, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10

Mathematics, 28.01.2021 06:10

History, 28.01.2021 06:10






