
Chemistry, 09.04.2021 21:00, bossninja97588
DO NOT ANSWER FOR THE POINTS ALSO NO RANDOM PICTURE OR LINKS
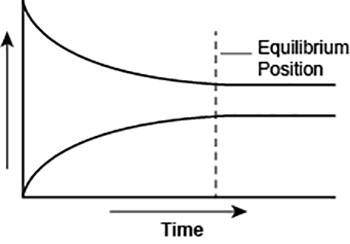
A student made a graph to show the chemical equilibrium position of a reaction. The student forgot to label the y-axis of the graph.
A graph is shown with two graph lines. One graph line starts at a higher position on the y axis and slopes downwards towards the right. The other graph line starts at a lower position on the y axis and slopes upwards towards the right. The two graph lines stop short of intersecting each other and continue as separate lines which gradually become straight and parallel to the x-axis. A vertical line is shown at a point where the two graph lines finally became parallel to the x-axis. This vertical line is labeled equilibrium. The title on the x-axis is Time and an arrow pointing towards the right is shown above Time. The title on the y axis is left blank.
What best explains the label that the student should use on the y-axis? (5 points)
a. Concentration, because as the amount of product decreases, the amount of reactant increases over time.
b. Reaction rate, as the rates of forward and backward reactions become equal at equilibrium.
c. Concentration, because the amounts of reactants and products remain constant after equilibrium is reached.
d. Reaction rate, as the rate of forward reaction increases and rate of backward reaction decreases over time.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, noathequeen
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
Do you know the correct answer?
DO NOT ANSWER FOR THE POINTS ALSO NO RANDOM PICTURE OR LINKS
A student made a graph to show the che...
Questions in other subjects:






Physics, 29.08.2019 12:50

World Languages, 29.08.2019 12:50

Mathematics, 29.08.2019 12:50

Business, 29.08.2019 12:50






