
Chemistry, 09.04.2021 16:30, kitttimothy55
En su cuaderno de química balancee las siguientes ecuaciones por el método redox. Suba su evidencia a este formulario
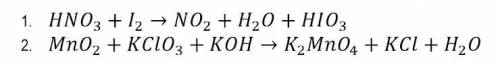

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:20, monsurviky
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 23.06.2019 01:30, oliviacolaizzi
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
Do you know the correct answer?
En su cuaderno de química balancee las siguientes ecuaciones por el método redox. Suba su evidencia...
Questions in other subjects:



Mathematics, 01.11.2019 17:31







Mathematics, 01.11.2019 17:31






