
Chemistry, 08.04.2021 22:20, cornpops4037
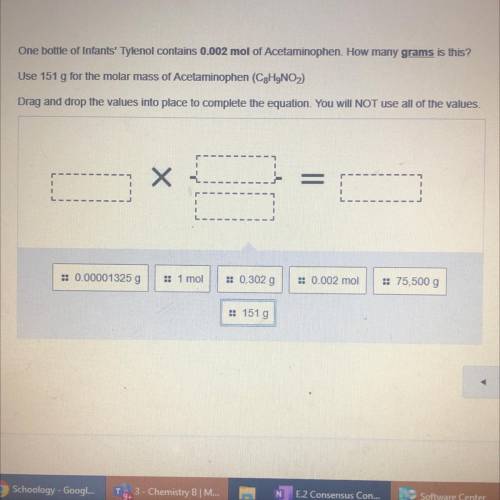
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151 g for the molar mass of Acetaminophen (C2H9NO)
Drag and drop the values into place to complete the equation. You will NOT use all of the values.
Х
= 0.00001325 g
1 moi
:: 0.302 g
: 0.002 mol
= 75,500 g
:: 151 g

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, officialrogerfp3gf2s
How to solve 4 nh3(g) + 5 o2(g) > 4 no(g) + 6 h2o(g) in chemistry
Answers: 1

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1
Do you know the correct answer?
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151...
Questions in other subjects:



Biology, 27.07.2019 01:30




Biology, 27.07.2019 01:30

Mathematics, 27.07.2019 01:30

History, 27.07.2019 01:30






