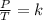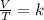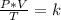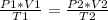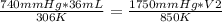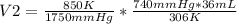Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, mariamakonteh31
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 10:00, ellaemtagedeane
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
Do you know the correct answer?
A gas has three initial measurements: temperature 306 K, pressure 740 mm Hg, and volume 36 mL. The g...
Questions in other subjects:



Mathematics, 20.12.2020 03:00

Physics, 20.12.2020 03:00