Calculate the number of moles or atoms in each of
the following quantities.
a. moles of...

Chemistry, 08.04.2021 18:20, greystokey
Calculate the number of moles or atoms in each of
the following quantities.
a. moles of each element in 3.35 moles of aspirin
(C9H8O4)
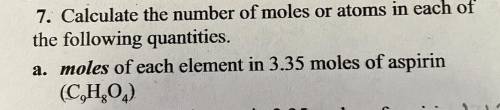

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
Do you know the correct answer?
Questions in other subjects:

English, 14.08.2020 21:01

Mathematics, 14.08.2020 21:01


English, 14.08.2020 21:01

History, 14.08.2020 21:01



Chemistry, 14.08.2020 21:01


Mathematics, 14.08.2020 21:01






