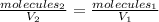Chemistry, 08.04.2021 08:40, brooke0713
Imagine that you have an ideal gas in a 5.80 L container, and that 2950 molecules of this gas collide with a square-centimetre area of the container at any given instant. If the volume is increased to 46.4 L at constant temperature, how many collisions will occur per square centimetre of this larger container?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2


Chemistry, 22.06.2019 10:30, perezanthony2403
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
Do you know the correct answer?
Imagine that you have an ideal gas in a 5.80 L container, and that 2950 molecules of this gas collid...
Questions in other subjects:


English, 26.09.2021 21:50

Mathematics, 26.09.2021 21:50



Engineering, 26.09.2021 21:50


Mathematics, 26.09.2021 21:50


History, 26.09.2021 21:50