
Chemistry, 08.04.2021 01:20, emmaja121003
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
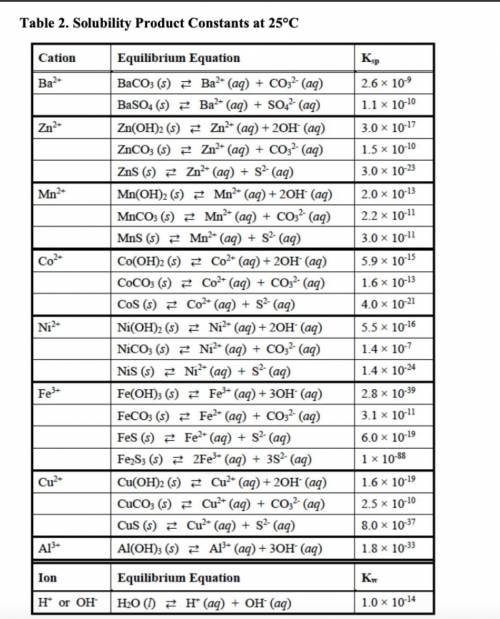
out the corresponding solubility equilibrium equations that would give rise to a precipitate with ammonia. Be careful to use the appropriate arrows (⇄ vs. →), depending on the compound’s solubility. Using the Ksp values provided in Table 2, predict whether it would form using 5 drops (0.25 mL) of 3 M NH3 and 1 mL of 0.10 M metal nitrate solution.
Your answer must include:
• The relevant chemical equation(s) showing dissociation or equilibrium of the
products into their ionic components.
• The Ksp values and equations
• Calculations showing how concentrations were determined
• Unique Qsp calculations
• A comparison of Qsp and Ksp, and discussion of what this comparison predicts
about precipitate (solid) formation.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 01:00, stefaniethibodeaux
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Do you know the correct answer?
For each of the seven metal cations (K+, Ba2+, Zn2+, Mn2+, Co2+, Ni2+, Fe3+), write
out the corresp...
Questions in other subjects:

English, 06.12.2019 22:31


Mathematics, 06.12.2019 22:31



Mathematics, 06.12.2019 22:31


Health, 06.12.2019 22:31


Mathematics, 06.12.2019 22:31






