
Chemistry, 22.09.2019 17:30, devbar3416
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water that results in a decrease in the water’s temperature. which of the following potential energy diagrams best illustrates the energy change of this dissolving process?
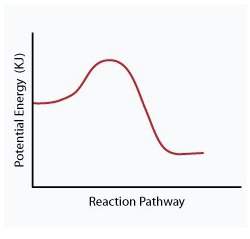

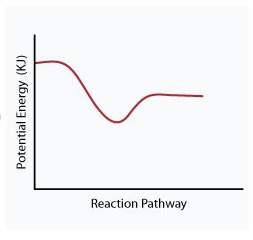
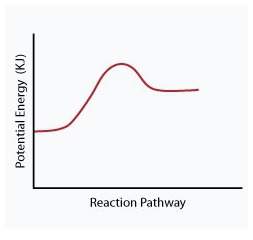
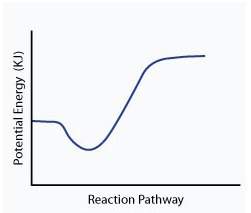

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 00:30, xoxokaydavis5837
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
Do you know the correct answer?
When a given reaction is conducted in a calorimeter, energy is absorbed from the surrounding water t...
Questions in other subjects:

Mathematics, 09.09.2021 19:30

Mathematics, 09.09.2021 19:30

English, 09.09.2021 19:30


Mathematics, 09.09.2021 19:30


Mathematics, 09.09.2021 19:30



World Languages, 09.09.2021 19:30






