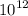Calculate the mass of vanadium(V) oxide (V2,05) that contains a trillion (1.00 x
) vanadium a...


Answers: 2
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 21:00, andrethisman88
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 29.10.2020 17:40

English, 29.10.2020 17:40

Chemistry, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40


Chemistry, 29.10.2020 17:40

Mathematics, 29.10.2020 17:40


Mathematics, 29.10.2020 17:40