

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, pinkycupcakes3oxbqhx
200. ml of 3.00 m nacl solution is diluted to a final volume of 500. ml. what is the molarity of the final solution?
Answers: 2

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 23.06.2019 02:50, giavanleer14
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 21:20, wsdafvbhjkl
Consider the total ionic equation below. what are the spectator ions in this equation? h+ and oh- h+ and ba2+cro2-4 and oh- cro2-4 and ba2+
Answers: 3
Do you know the correct answer?
34 g of O2 are reacted with excess Cs, causing a production of 199 g of Cs2O. What is the percent yi...
Questions in other subjects:



Mathematics, 25.04.2020 19:48

English, 25.04.2020 19:48







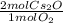 = 2.125 mol Cs₂O
= 2.125 mol Cs₂O



