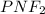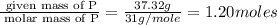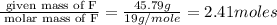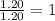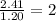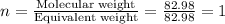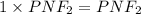Chemistry, 06.04.2021 22:50, josephvcarter
A compound is found to contain 37.32 % phosphorus , 16.88 % nitrogen , and 45.79 % fluorine by
mass.
Question 1: The empirical formula for this compound is :
Question 2: The molar mass for this compound is 82.98 g/mol.
The Molecular formula for this compound is:

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2
Do you know the correct answer?
A compound is found to contain 37.32 % phosphorus , 16.88 % nitrogen , and 45.79 % fluorine by
mass...
Questions in other subjects:



Health, 20.10.2021 22:50

Biology, 20.10.2021 22:50



Social Studies, 20.10.2021 22:50



SAT, 20.10.2021 22:50