

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, markmlg122
Two things that biomedical has invented or innvated
Answers: 1



Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
Do you know the correct answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions in other subjects:








English, 14.01.2020 17:31

English, 14.01.2020 17:31

English, 14.01.2020 17:31


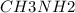 ) with 0.493 M
) with 0.493 M 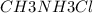 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?



