
Chemistry, 06.04.2021 20:00, Jackiecroce12
Find [H+] of a 0.056 M hydrofluoric acid solution. Ka = 1.45 x 10-7

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1
Do you know the correct answer?
Find [H+] of a 0.056 M hydrofluoric acid solution. Ka = 1.45 x 10-7...
Questions in other subjects:

Chemistry, 08.08.2019 04:30










![[H^+]](/tpl/images/1242/1877/07acb.png) of 0.056 M HF solution is
of 0.056 M HF solution is 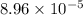
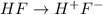
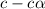
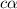
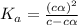
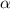 = ?
= ?
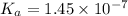
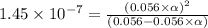
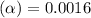
![[H^+]=c\times \alpha](/tpl/images/1242/1877/4fc41.png)
![[H^+]=0.056\times 0.0016=8.96\times 10^{-5}](/tpl/images/1242/1877/53c42.png)




