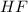Hydrogen fluoride reacts with ammonia in an acid-base reaction:
HF (aq) + NH3 (aq) -->
<...

Chemistry, 05.04.2021 07:50, SearchLight
Hydrogen fluoride reacts with ammonia in an acid-base reaction:
HF (aq) + NH3 (aq) -->
a. Determine the products made by completing the reaction above.
b. Identify and label one of the conjugate acid-base pairs.
Please I will give brainliest I’m stuck

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, monnn91351
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2


Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 23.06.2019 13:30, leahpartaka03
Which correctly identifies the parts of a transverse wave? a: crest b: amplitude c: wavelength d: trough a: trough b: amplitude c: crest d: wavelength a: trough b: amplitude c: wavelength d: crest a: crest b: amplitude c: trough d: wavelength
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 18.03.2021 02:40

Geography, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Health, 18.03.2021 02:40



Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40


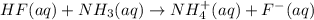
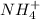 : conjugate acid of
: conjugate acid of 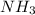
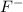 : conjugate base of
: conjugate base of