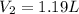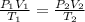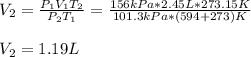Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 04:40, yayamcneal05
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
Do you know the correct answer?
What would be the volume in liters of an 2.45 liter sample of gas at 594 °C
and 156 kPa if conditio...
Questions in other subjects:

English, 05.03.2021 19:50


History, 05.03.2021 19:50

English, 05.03.2021 19:50




History, 05.03.2021 19:50

Social Studies, 05.03.2021 19:50