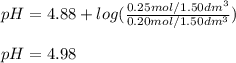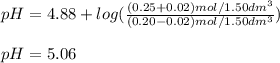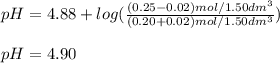A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate (CH3CH2COONa) in 1.50 dm3.
What is the pH of this buffer?
Enter your answer using two decimal places.
What is the pH of the buffer after the addition of 0.02 mol of NaOH?
What is the pH of the buffer after the addition of 0.02 mol of HI?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, ulilliareinhart2
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 08:00, tchase0616
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1


Do you know the correct answer?
A buffer solution contains 0.20 mol of propionic acid (CH3CH2COOH) and 0.25 mol of sodium propionate...
Questions in other subjects:





Mathematics, 30.01.2020 13:58

History, 30.01.2020 13:58

Chemistry, 30.01.2020 13:58

History, 30.01.2020 13:58

Mathematics, 30.01.2020 13:58

Mathematics, 30.01.2020 13:58


![pH=pKa+log(\frac{[base]}{[acid]} )](/tpl/images/1238/6169/33848.png)