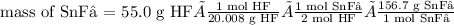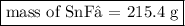Chemistry, 02.04.2021 17:40, aliceotter2007
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is prepared according
to the following equation:
Sn(s) + 2HF(aq) → SnF2(aq) + H2(g)
How many grams of tin(II) fluoride can be produced
from 55.0 g of hydrogen fluoride if there is plenty of tin
available to react?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1


Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
Do you know the correct answer?
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is pre...
Questions in other subjects:

Mathematics, 31.08.2020 17:01

Physics, 31.08.2020 17:01

English, 31.08.2020 17:01


History, 31.08.2020 17:01