
Chemistry, 02.04.2021 08:10, smithmorgan773p35885
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced equation, which reactant is the limiter and how many moles of excess reactant would remain after the reaction is completed? 2 Cu + S --> Cu2S
Cu is limiting and 1.00 mole of excess S remain
S is limiting and 1.00 moles of excess Cu remain
S is limiting and 4.00 moles of excess Cu remain
Cu is limiting and 4.00 moles of excess S remain

Answers: 2
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3
Do you know the correct answer?
If 10.00 moles of copper are reacted with 6.00 moles of sulfur according to the following balanced e...
Questions in other subjects:




Mathematics, 13.11.2020 01:00


Mathematics, 13.11.2020 01:00

History, 13.11.2020 01:00


Biology, 13.11.2020 01:00


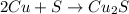
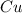 require 1 mole of
require 1 mole of 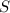
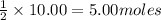 of
of 



