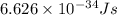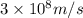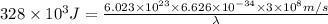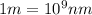Chemistry, 02.04.2021 02:10, vivianfling
1. Chlorofluorocarbons (CFCs) A carbon-chlorine bond in the CFC molecule can be broken by sunlight, leaving a highly reactive free radical which then goes on to destroy the surrounding ozone molecules. The energy of a C-Cl bond is 328 kJ/mole. Calculate the wavelength of light needed to break a bond in a single molecule. In which region of the spectrum (infrared, visible, UV) does this wavelength fall

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
1. Chlorofluorocarbons (CFCs) A carbon-chlorine bond in the CFC molecule can be broken by sunlight,...
Questions in other subjects:


History, 05.03.2021 21:30

French, 05.03.2021 21:30

History, 05.03.2021 21:30



Biology, 05.03.2021 21:30

Mathematics, 05.03.2021 21:30

Biology, 05.03.2021 21:30


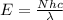
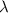 = wavelength of the wave
= wavelength of the wave