
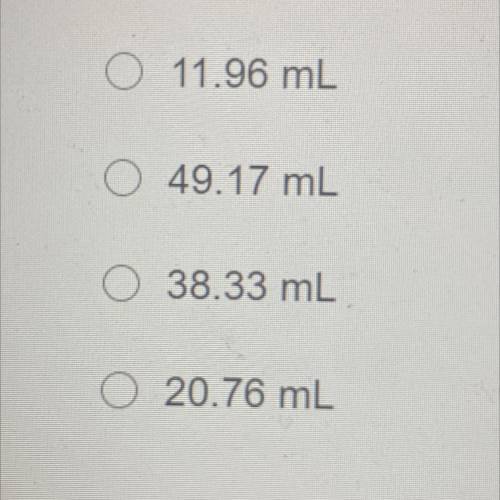
An unopened soda bottle contains 46.0mL of gas confined at a pressure of 1.30 atm at a temperature of 5.0°C. If the bottle is
dropped into a lake and sinks to a depth at which the pressure is 2.85 atm and temperature is 2.09°C, what will the volume of the
gas in the bottle be?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:40, yfgkeyonna
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30, UaRemomGAY
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:30, kaytonleeb
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2
Do you know the correct answer?
An unopened soda bottle contains 46.0mL of gas confined at a pressure of 1.30 atm at a temperature o...
Questions in other subjects:



Mathematics, 21.02.2022 15:10

Mathematics, 21.02.2022 15:10

Mathematics, 21.02.2022 15:10

Mathematics, 21.02.2022 15:10


Mathematics, 21.02.2022 15:10


Mathematics, 21.02.2022 15:10






