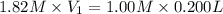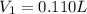A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring out some aqueous silver nitrate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the silver nitrate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
Do you know the correct answer?
A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring...
Questions in other subjects:



Social Studies, 22.11.2020 06:40

Mathematics, 22.11.2020 06:40


Computers and Technology, 22.11.2020 06:40

Mathematics, 22.11.2020 06:40




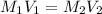
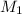 = molarity of stock silver nitrate solution = 1.82 M
= molarity of stock silver nitrate solution = 1.82 M
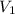 = volume of stock silver nitrate solution = ?
= volume of stock silver nitrate solution = ?