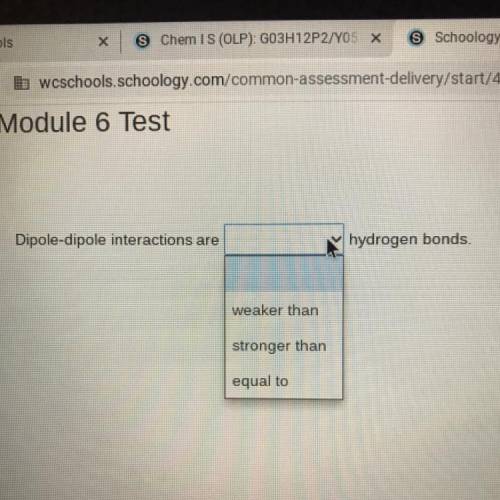
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.
...

Chemistry, 02.04.2021 01:00, postorivofarms
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2
Do you know the correct answer?
Questions in other subjects:



History, 29.07.2019 21:00

Mathematics, 29.07.2019 21:00

Chemistry, 29.07.2019 21:00


History, 29.07.2019 21:00

History, 29.07.2019 21:00

History, 29.07.2019 21:00







