
Chemistry, 01.04.2021 21:50, JamesLachoneus
Ok, so I have relised that ALOT of people have been living in a "what if" world". I have too. Its a world that I advise you to try to break free from. What if they don't like me? what if it looks stup. id? What if .? There are soo many what if's. Well, what if you die tomorrow? What if u regret never doing it? Now of course this doesn't mean go do something that hurts u or anyone else. But I wanted to say this because I wanted ppl to relise what I have relised: Of we keep letting our fears control us, then we will accomplish nothing. And I uderstand that not alot of ppl are even going to read this. I probably wouldn't. "Too many worlds". But if u are the .1119% that did, stop saying what if, and just go do it. What if. all you dreams com true???


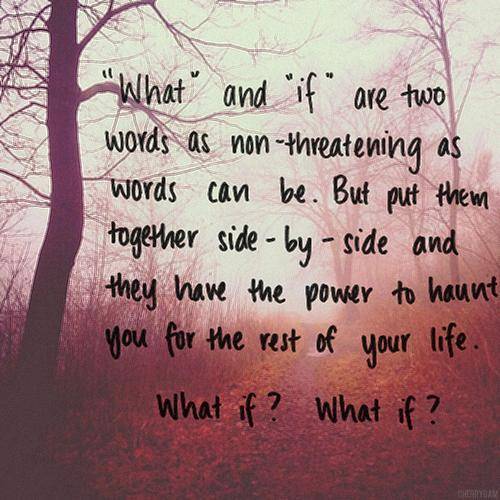

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3


Chemistry, 23.06.2019 00:40, joe7977
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
Do you know the correct answer?
Ok, so I have relised that ALOT of people have been living in a "what if" world". I have too. Its a...
Questions in other subjects:

Computers and Technology, 17.09.2019 10:30



Social Studies, 17.09.2019 10:30



Chemistry, 17.09.2019 10:30

Advanced Placement (AP), 17.09.2019 10:30

History, 17.09.2019 10:30

English, 17.09.2019 10:30






