
Chemistry, 01.04.2021 19:20, bryanmcmillianjr
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of 0.100 M NaOH. The student then titrates in 11.0 mL of 0.100 M HCl to the solution containing the neutralized unknown acid (HCl was delivered from a second buret). The pH of the resulting solution is pH

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1



Chemistry, 22.06.2019 22:30, teagan56
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
Do you know the correct answer?
A student titrates an unknown weak acid, HA, to a pale pink phenolphthalein endpoint with 25.0 mL of...
Questions in other subjects:

Business, 23.07.2019 06:30

History, 23.07.2019 06:30




English, 23.07.2019 06:30


Mathematics, 23.07.2019 06:30



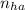 = 2.5 mmoles ------ 1
= 2.5 mmoles ------ 1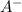 + HCL ---------> HA + C
+ HCL ---------> HA + C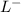
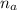 = 1.1 mmoles --------- 2
= 1.1 mmoles --------- 2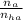 = 1.1 / 2.5 ≈ 1/2 ( this show that when the pH = 4.7 approximately half of the A^- have been converted to HA )
= 1.1 / 2.5 ≈ 1/2 ( this show that when the pH = 4.7 approximately half of the A^- have been converted to HA ) 




