Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
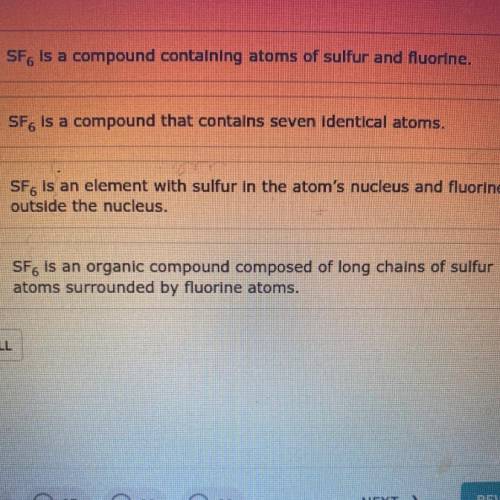
Do you know the correct answer?
Sulfur hexafluoride, SF6, is a very stable, nonflammable gas at room temperature. It
is used in man...
Questions in other subjects:



Mathematics, 23.07.2020 01:01

Health, 23.07.2020 01:01


Mathematics, 23.07.2020 01:01