
Chemistry, 31.03.2021 20:50, alexkrol10
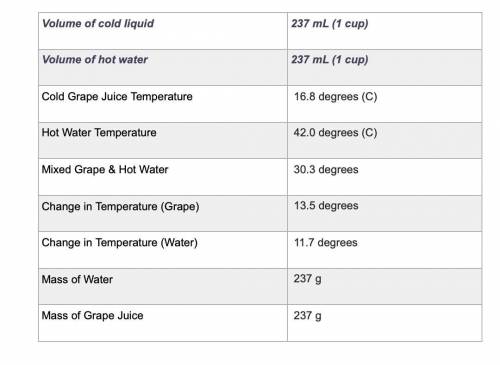
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the specific heat for the liquid you selected. Use the equation qwater = m × c × ΔT to calculate the heat lost by the hot water. Show your work using the problem-solving method shown in previous rubrics.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, hdjsjfjruejchhehd
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 10:30, Clivensp5
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils. the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
Do you know the correct answer?
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the speci...
Questions in other subjects:




Mathematics, 20.06.2020 02:57

Mathematics, 20.06.2020 02:57



Geography, 20.06.2020 02:57







