
Chemistry, 31.03.2021 18:50, tlperez1230
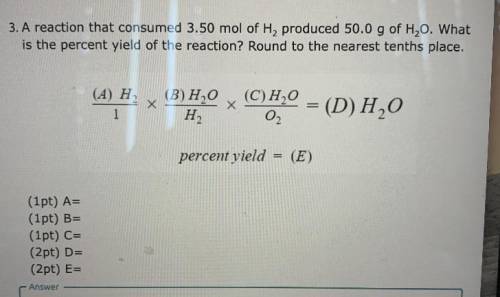
A reaction that consumed 3.50 mol of H2 produced 50.0 g of H20. What is the percent yield of the reaction? Round to the nearest tenths place.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 20:00, teacherpreacher
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 23.06.2019 03:30, tamariarodrigiez
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
Do you know the correct answer?
A reaction that consumed 3.50 mol of H2 produced 50.0 g of H20. What
is the percent yield of the re...
Questions in other subjects:


English, 07.10.2019 10:30


Social Studies, 07.10.2019 10:30






English, 07.10.2019 10:30






