
Chemistry, 30.03.2021 22:00, Lollipop1287
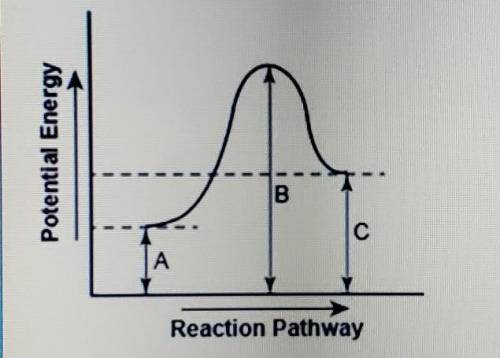
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
part 2: describe how the curve will look if the reaction was exothwrmic. be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, bigwaYne
Imagine that twenty i. u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2



Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
Do you know the correct answer?
The diagram shows the potential energy changes for a reaction pathway.
part 1: describe how you can...
Questions in other subjects:

History, 03.09.2019 23:10


History, 03.09.2019 23:10












