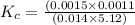Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) + H2O(I) rightarrow F-(aq) + H3O+(aq)
At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition:
Compound Amount
HF 1.62 g
H2O 516 g
F- 0.163 g
H3O+ 0.110 g
Calculate the value of the equilibrium constant for this reaction.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
Do you know the correct answer?
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) +...
Questions in other subjects:



Mathematics, 11.01.2021 17:40

Business, 11.01.2021 17:40

Mathematics, 11.01.2021 17:40


Mathematics, 11.01.2021 17:40

Biology, 11.01.2021 17:40



 =
= 
 =
= 
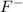 =
= 
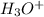 =
= 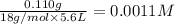

![K_c=\frac{[F^-]\times [H_3O^+]}{[HF]\times [H_2O]}](/tpl/images/1230/8174/06b96.png)